Thin films
Information guide
Innovation often involves new materials, but these are far from being all new molecules or revolutionary alloys. In most cases, innovation involves improving the qualities of existing materials by treating their surface, either by adding one or more layers or by changing the physical characteristics of the surface. This means, for example, maintaining the mechanical qualities of a material while improving its resistance to corrosion by modifying its surface. The scope of use of vacuum in the treatment of materials is constantly growing because these treatments offer qualities of adhesion, cleanliness and repeatability, while at the same time making it possible to avoid the problems of effluent from wet deposits. Moreover, these are efficient deposits, i.e. requiring only small quantities of precursors (gas, liquid or solid materials).
Categories of vacuum surface treatments
Vacuum treatment refers to the fact that the parts to be treated are inside a pumped enclosure, and are thus under either primary or secondary vacuum. Whether it is a question of surface modification or surface deposition, the thicknesses involved are always low, from a few nanometers to a few microns for thin film deposition.
Physical Vapor Deposition (PVD) :
The starting point is a solid precursor, placed inside the enclosure, which is brought to the vapour phase. This vapour, by condensing on the surface of the parts, will create a layer. Depending on the energy of the evaporated particles, the main methods are :
- Thermal evaporation (Joule effect);
- Electron gun evaporation (e-Gun or e-Beam)
- Sputtering
Thermal evaporation (Joule effect evaporation, PVD, 10-4 to 10-6 mbar) :
The principle is simple: the material to be evaporated is heated in a secondary vacuum until its vapour phase is obtained. The vapour condenses on the parts, creating a thin layer (typically < 1µ). To do this, a filament, a crucible or a boat source made of refractory material (W, Mo, Ta, BN, Al2O3) is used in which the material to be evaporated is deposited (figure 1).
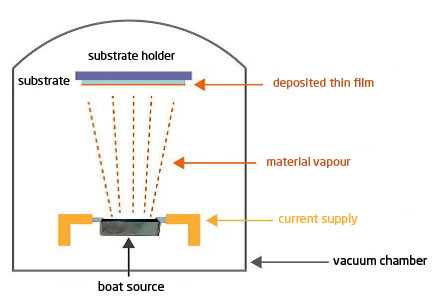
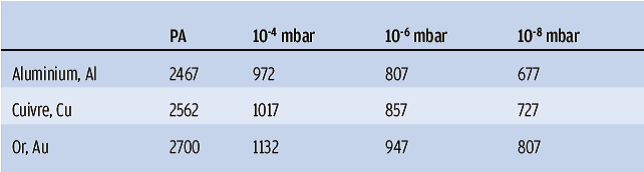
It should be noted that the vapour phase of materials does not occur at the same temperature under secondary vacuum as at atmospheric pressure. Vaporisation under vacuum therefore takes place at temperatures that are generally well below the known melting point of the PA. Each material has its own vaporisation temperature, which is a function of the pressure (called vapour pressure): the lower the vacuum, the lower the vaporisation temperature (table 1).
Being in a secondary vacuum with less risk of collision (high lpm), there is no chemical alteration by water vapour or oxygen in residual form, and low thickness (typically <1µ) and high purity deposits can be achieved.
Advantages :
- simplicity of implementation (lower cost of equipment)
- wide choice of materials
- no waste
Disadvantages :
- sometimes high temperature incompatible with certain organic parts
- no acceleration of particles, therefore average adhesion
- no possibility of evaporating refractory materials
Examples of applications:
- aluminium deposition of reflective layers (car headlights, telescope mirrors) ;
- zinc plating on metal sheets (replacement of electrolytic baths);
- Aluminium and ZnS for the creation of holograms;
- Cr and SiO2 for "head-up" reading screens in cars and helmet visors
Electron gun evaporation (e-Beam, PVD, 10-4 to 10-6 mbar) :
The material to be evaporated is placed in a crucible, and will be bombarded by an electron beam heating its surface. Thanks to the energy and concentration of the beam, the deposits are made at a relatively high speed. The principle of the vapour phase is the same as for Joule Effect evaporation (figure 2).
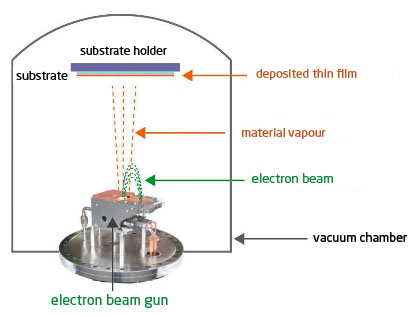
Advantages : thickness control is extremely precise (no inertia) which offers a very high reproducibility of the deposited layers. The power of the electron beam allows the evaporation of refractory metals, alloys and oxides. The use of rotating multi-cuspets allows the multiplication of materials in situ, and is therefore very suitable for multi-layers.
Disadvantages : Depositions are only made in 2 dimensions, therefore on flat substrates. Moreover, the deposition area is limited to the coverage of the evaporation cone.
Examples of applications:
- SiO2 and TiO2 in optics (filters and ophthalmic lenses);
- stacking of layers for semiconductors (Ni and Au)
Sputtering (PVD, 10-1 to 10-3 mbar) :
The principle is to use the energy of a plasma (partially ionised gas) on the surface of a target (cathode) to pull out the atoms of the material one by one and deposit them on the substrate (figure 3).
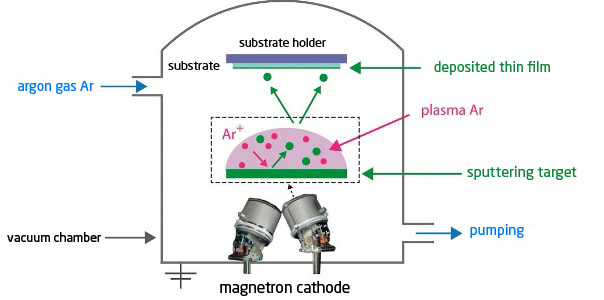
To do this, a plasma is created by ionising a pure gas (usually Argon) using a potential difference (DC or pulsed DC), or electromagnetic excitation (MF, RF). This plasma is composed of Ar+ ions which are accelerated and confined around the target by the presence of a magnetic field. Each ionised atom, upon impacting the target, transfers its energy to the target and tears off an atom, which has sufficient energy to be projected towards the substrate.
The plasma is created at relatively high pressures (10-1/10-3 mbar), but it is necessary to start from a lower pressure before the introduction of Argon, to avoid contamination from residual gases in the chamber.
Advantages : the diversity of target shapes (flat or tubular) and materials used allows all types of thin films, including alloys, to be created in the same run. The adhesion is good and the method allows for roll-on deposition.
Disadvantages : the machine cost is often high (cathode and power supply cost)
There are many applications :
- Zinc anticorrosion deposits on steel
- metallized films on unwinding
- Tungsten deposits for hardening on crankshaft bearings
- Vanadium base on thermal solar panels
- athermal deposits such as SiOx, TiOx, Sn, Ag on windows in the building industry
_1673427121.png)




_1663761381.jpg)




